Introduction: CPX-351 (Vyxeos®; daunorubicin and cytarabine liposome for injection), a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar ratio, has been approved by the US FDA and the EMA for the treatment of adults with newly diagnosed, therapy-related AML or AML with myelodysplasia-related changes. The primary analysis of the pivotal phase 3 study (NCT01696084) that formed the basis for these approvals evaluated patients aged 60 to 75 years with newly diagnosed high-risk/secondary AML and found that, with a median follow-up of 20.7 months, CPX-351 significantly improved median overall survival (OS) versus conventional 7+3, with a comparable safety profile. Final 5-year follow-up results recently reported demonstrated that the OS benefit was maintained. To evaluate both quality and quantity of life, we conducted a Q-TWiST analysis of the phase 3 study to compare survival between patients receiving CPX-351 versus 7+3.
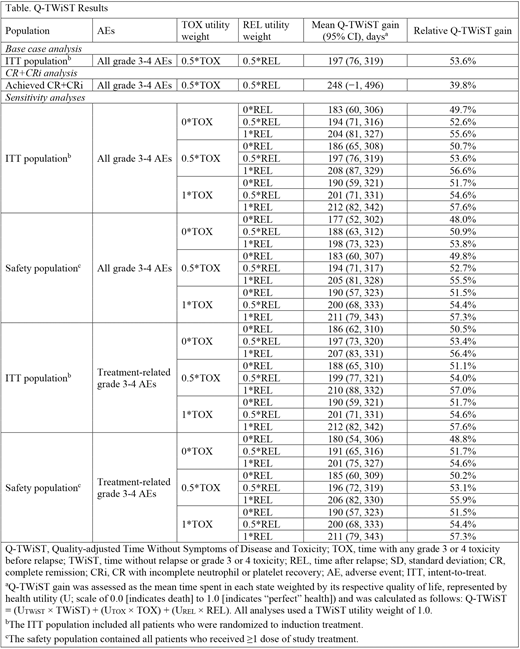
Methods: Q-TWiST is used to evaluate outcomes in oncology trials by measuring how much of the survival improvement time is spent with toxicities, how much after disease progression or relapse, and how much is valuable time (ie, Time Without Symptoms of Disease and Toxicity [TWiST]). For this analysis, the OS for each patient from the 5-year follow-up analysis was partitioned into 3 health states: TOX (time before response plus any additional time with a grade 3 or 4 toxicity), TWiST (time without relapse or grade 3 or 4 toxicity), and REL (time after relapse). Q-TWiST gain was assessed as the mean time spent in each state weighted by its respective quality of life, represented by health utility (U; scale of 0.0 [indicates death] to 1.0 [indicates "perfect" health]). Q-TWiST gain was calculated as follows: Q-TWiST = (UTWiST × TWiST) + (UTOX × TOX) + (UREL × REL). The base case scenario used the intent-to-treat population, any grade 3 or 4 toxicities, TOX and REL utility weights of 0.5, and a TWiST utility weight of 1.0. Sensitivity analyses were performed for all treated patients (CPX-351: n = 153; 7+3: n = 151) and the intent-to-treat population, any and treatment-related grade 3 or 4 toxicities, and TOX and REL utility weights of 0, 0.5, and 1.0. A variation of the base case scenario was also performed for the subset of patients who achieved complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CR+CRi; CPX-351: n = 73; 7+3: n = 52).
When comparing across populations or studies, reporting relative Q-TWiST gains is an effective measure for evaluating clinical benefit (ie, Q-TWiST gains compared to a control; Solem CT, et al. Expert Rev Pharm Out 2018). A relative Q-TWiST gain of 15% or greater is considered a clinically important difference (CID) in oncology studies (Revicki DA, et al. Qual Life Res 2006). The relative Q-TWiST gain was calculated using the following equation: Q-TWiST difference ÷ mean OS of control arm × 100.
Results: In total, 309 patients were randomized to CPX-351 (n = 153) or 7+3 (n = 156). In the base case scenario, the means difference (95% CI) for CPX-351 versus 7+3 was 183 days (60, 306) for the TWiST state, 7 days (−63, 78) for the TOX state, and 22 days (5, 38) for the REL states. The resulting means difference (95% CI) for Q-TWiST gain was 197 days (76, 319) for CPX-351 versus 7+3, and the relative Q-TWiST gain was 53.6%. Among patients who achieved CR or CRi, the means difference (95% CI) for Q-TWiST gain was 248 days (−1, 496) for CPX-351 versus 7+3, and the relative Q-TWiST gain was 39.8%. Both relative Q-TWiST gains were considerably above the standard CID of 15% for oncology. Across the various sensitivity analyses, the relative Q-TWiST gains for CPX-351 versus 7+3 varied from 48.0% to 57.6%, remaining all well above the standard CID threshold (Table).
Conclusions: Results of this post hoc analysis suggest the survival benefit with CPX-351 for patients with high-risk/secondary AML are mostly from valuable time (TWiST), thus supporting the clinical benefit for patients. The relative Q-TWiST gains were well above what is considered CID (15%) in the cancer literature and were maintained across various sensitivity analyses, supporting the robustness of the benefit. In the absence of direct measures of quality of life, these results can be used together with the antileukemia effect when considering treatment options for this patient population.
Cortes:Daiichi Sankyo: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding; BioPath Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Telios: Research Funding; Astellas: Research Funding; Amphivena Therapeutics: Research Funding; Arog: Research Funding; BiolineRx: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; Merus: Research Funding; Immunogen: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Takeda: Consultancy, Research Funding. Lin:Aptevo: Research Funding; Abbvie: Research Funding; Gilead Sciences: Research Funding; Genetech-Roche: Research Funding; Incyte: Research Funding; Celyad: Research Funding; Celgene: Research Funding; Jazz: Research Funding; Mateon Therapeutics: Research Funding; Ono Pharmaceutical: Research Funding; Bio-Path Holdings: Research Funding; Astellas Pharma: Research Funding; Prescient Therapeutics: Research Funding; Pfizer: Research Funding; Trovagene: Research Funding; Tolero Pharmaceuticals: Research Funding; Seattle Genetics: Research Funding. Uy:Daiichi Sankyo: Consultancy; Pfizer: Consultancy; Agios: Consultancy; Genentech: Consultancy; Jazz Pharmaceuticals: Consultancy; Astellas Pharma: Honoraria. Ryan:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Faderl:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Lancet:Abbvie: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria; Astellas Pharma: Consultancy; Celgene: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; ElevateBio Management: Consultancy; Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal